
Leader: dr Monika Szymańska-Chargot
Team: mgr Monika Chylińska, dr inż. Piotr M. Pieczywek, mgr Luiza Wątróbka, dr hab. Artur Zdunek
The aim of the task is to determine the changes in the content and localization of the polysaccharides in the cell wall tissue of developing tomato fruit (Lycopersicon esculentum Mill.) using Raman imaging. This objective is related to the research hypothesis, which says that the chemical composition of cell walls and spatial distribution of polysaccharides changes during the growth of fruit and vegetables, which has an impact on the integrity of the cell wall.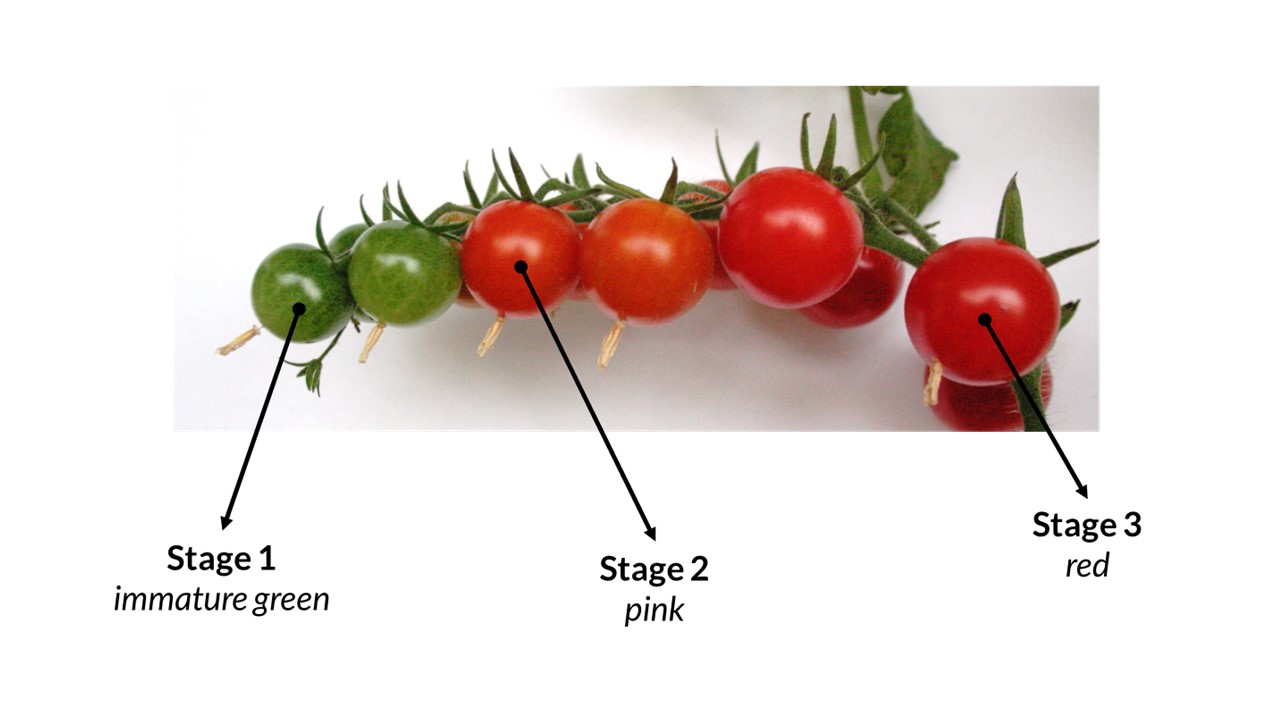
The most often method used for the localization of plant cell wall polysaccharides is imaging using antibodies. Immunocytochemistry is a technique used to assess the presence of a specific protein or antigen in cells by use of a specific antibody, which binds to it, thereby allowing visualization and examination under a microscope. Antigens may be primarily protein, but also polysaccharides and nucleic acids. However, these methods are very time consuming, quite expensive and selective.
The alternative could be Raman mapping. This method is non-destructive and does not require extensive sample pretreatment. Moreover, an advantage of Raman microspectroscopy is the possibility of acquiring the complete information about the spatial distribution of chemical components during a single measurement in form of a hyperspectral image
Raman mapping has been one of the most developed Raman spectroscopy techniques in recent years. In this method the Raman spectra are collected in a number of spatially separated points on the sample surface. As a result, so called chemical image is obtained. Raman images of cell walls components are generated simply by integrating over a certain wavenumber region highlighting substantial changes in the amount and localization of the main cell wall polysaccharides. 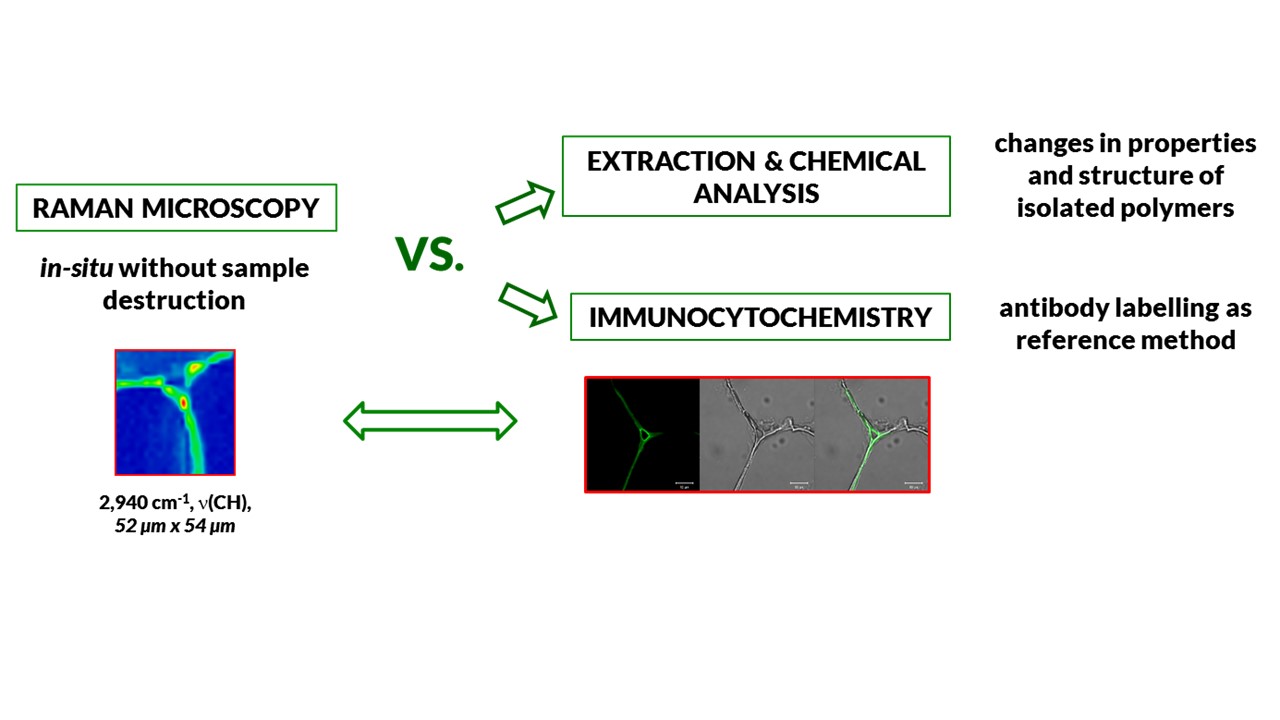
During the task realization Raman maps will be used to investigate the spatial distribution of plant cell walls components. The results obtained from the Raman microspectroscope and confocal microscopy (immunocytochemistry) as well as results of chemical analysis and infrared spectra will be collate to obtain information about change cell walls polysaccharides (their amount and distribution) during growth and maturation the tomato fruit. Additionally, Raman imaging method will be validated by the antibodies labeling method, for example for pectins localization.
References
- Chylińska M, Szymańska-Chargot M, Zdunek A (2014) Imaging of polysaccharides in the tomato cell wall with Raman microspectroscopy. Plant Meth 10: 14.
- Gierlinger N, Keplinger T, Harrington M (2012) Imaging of plan cell walls by confocal Raman microscopy. Nat Protoc 7:1694-1708.
- Qin J, Chao K, Kim MS (2011) Investigation of Raman chemical imaging for detection of lycopen changes in tomatoes during postharvest ripening. J Food Eng 107: 277-288.
- Richter S, Mussig J, Gierlinger N (2011) Functional plant cell wall design revealed by the Raman imaging approach. Planta 233:768–772.
- Szymańska-Chargot M, Zdunek A (2013) Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables Along a Fraction Process. Food Bioph 8: 29-42.
- Szymańska-Chargot M, Chylińska M, Kruk,B, Zdunek A (2015) Combining FT-IR spectroscopy and multivariate analysis for qualitative and quantitative analysis of the cell wall composition changes during apples development. Carbohydr Pol 115: 93-103.
- Szymańska-Chargot M., Pieczywek P. M., Chylińska M., Zdunek A. (2016a) Hyperspectral image analysis of Raman maps of plant cell walls for blind spectra characterization by nonnegative matrix factorization algorithm. Chemometr Intell Lab 151:136-145
- Szymańska-Chargot M., Chylińska M., Pieczywek P. M., Rösch P., Schmitt M., Popp J., Zdunek A. (2016b) Raman imaging of changes in the polysaccharides distribution in the cell wall during apple fruit development and senescence. Planta (2016b), DOI:10.1007/s00425-015-2456-4
